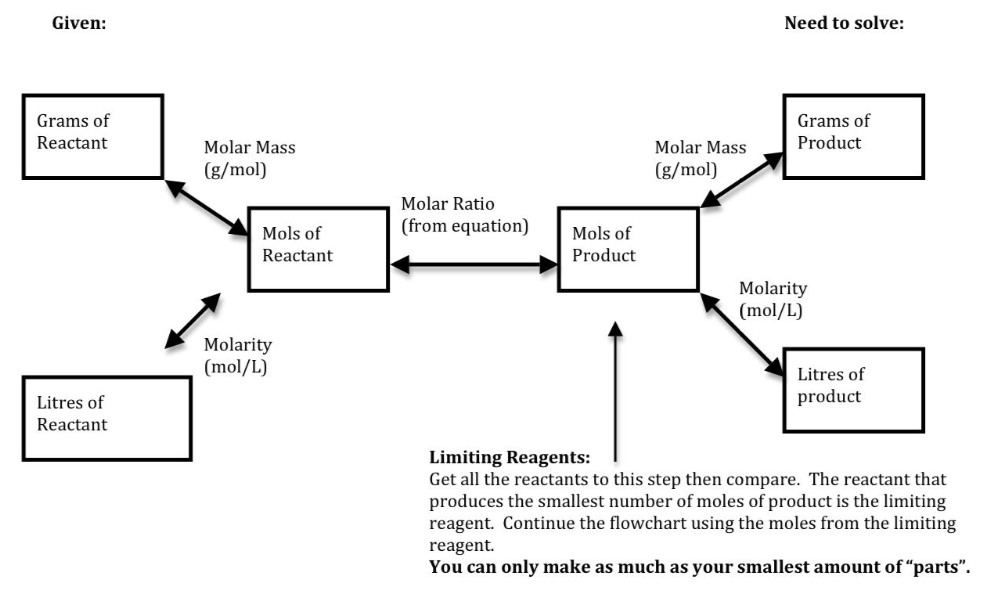
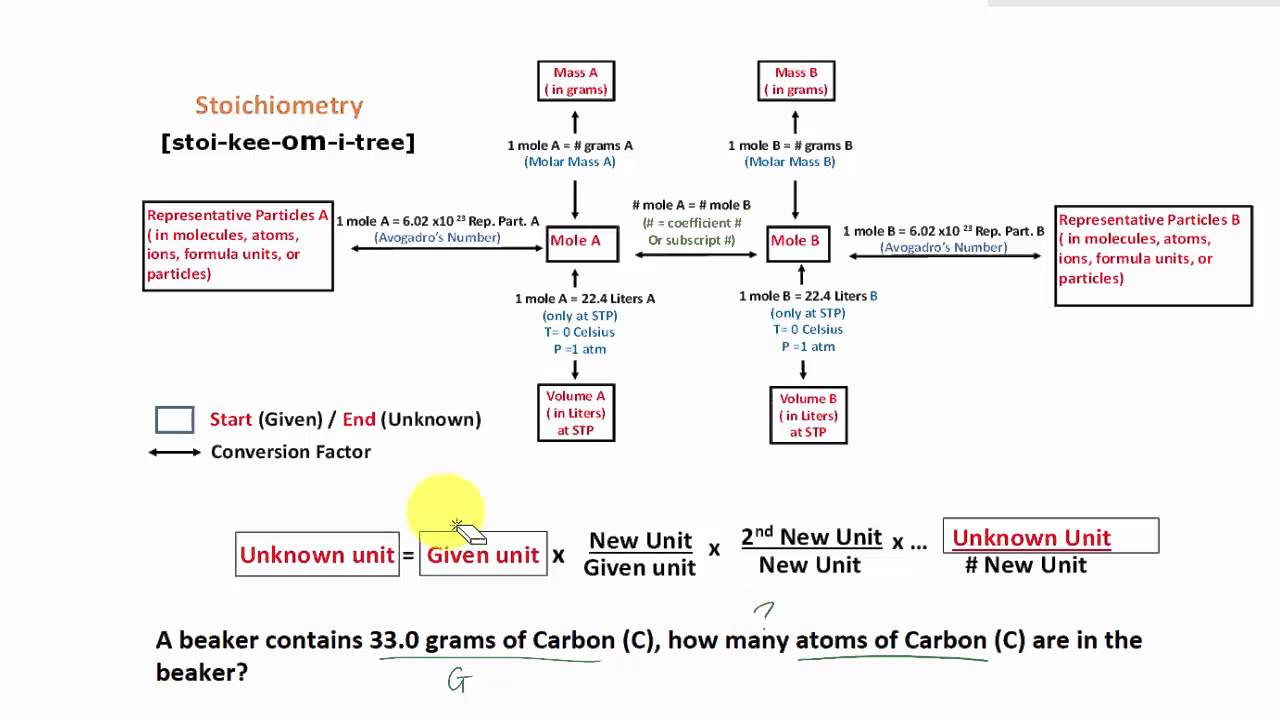
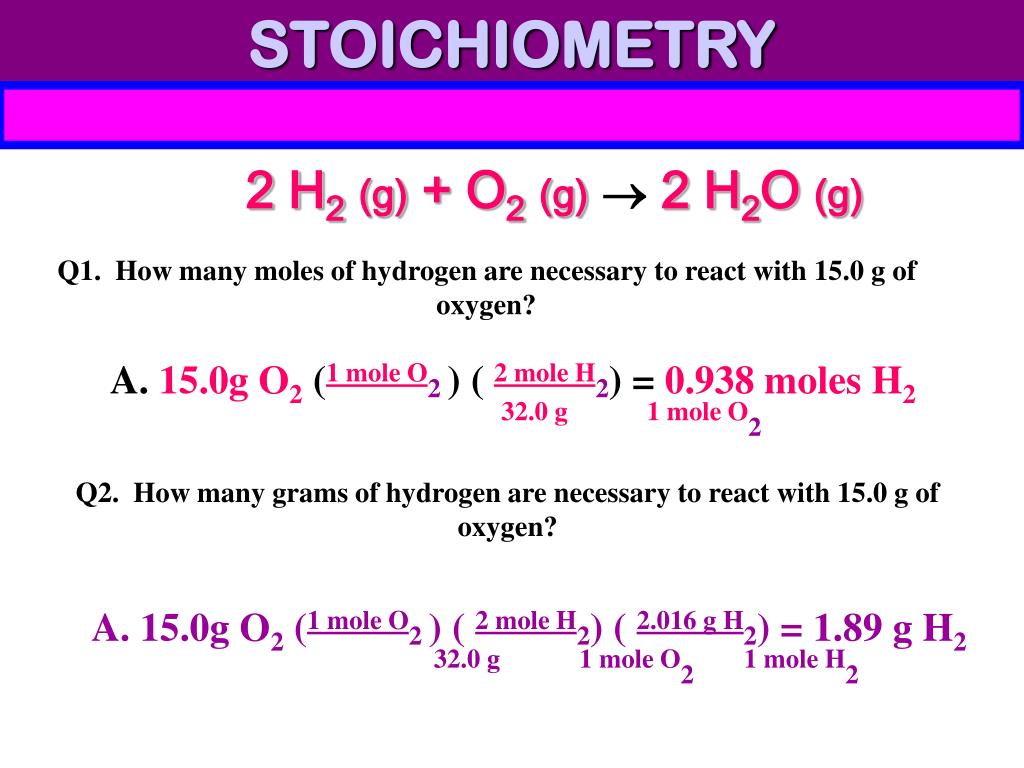
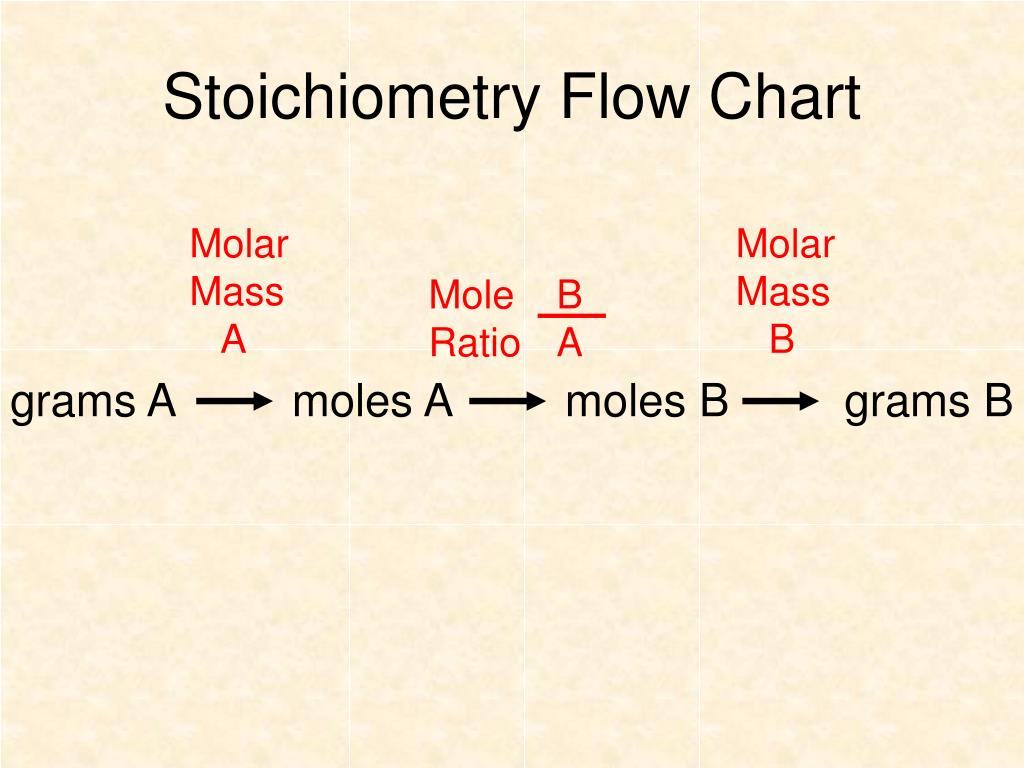
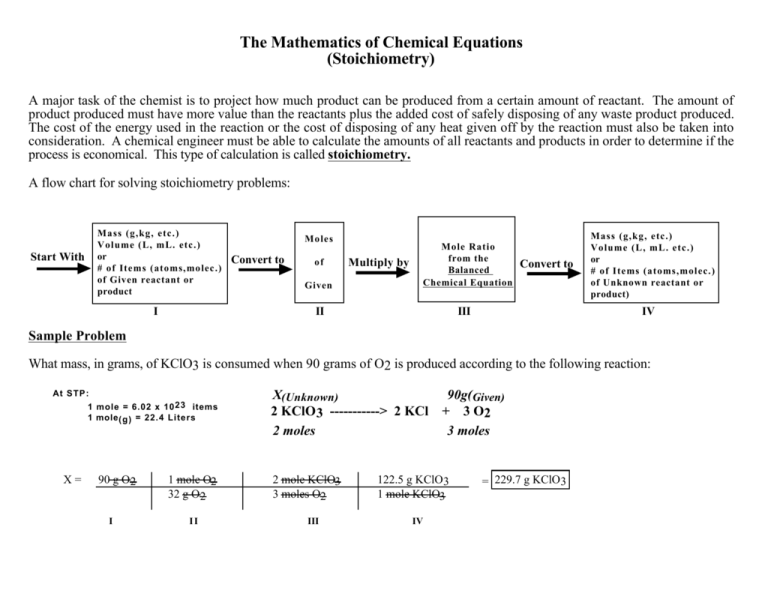
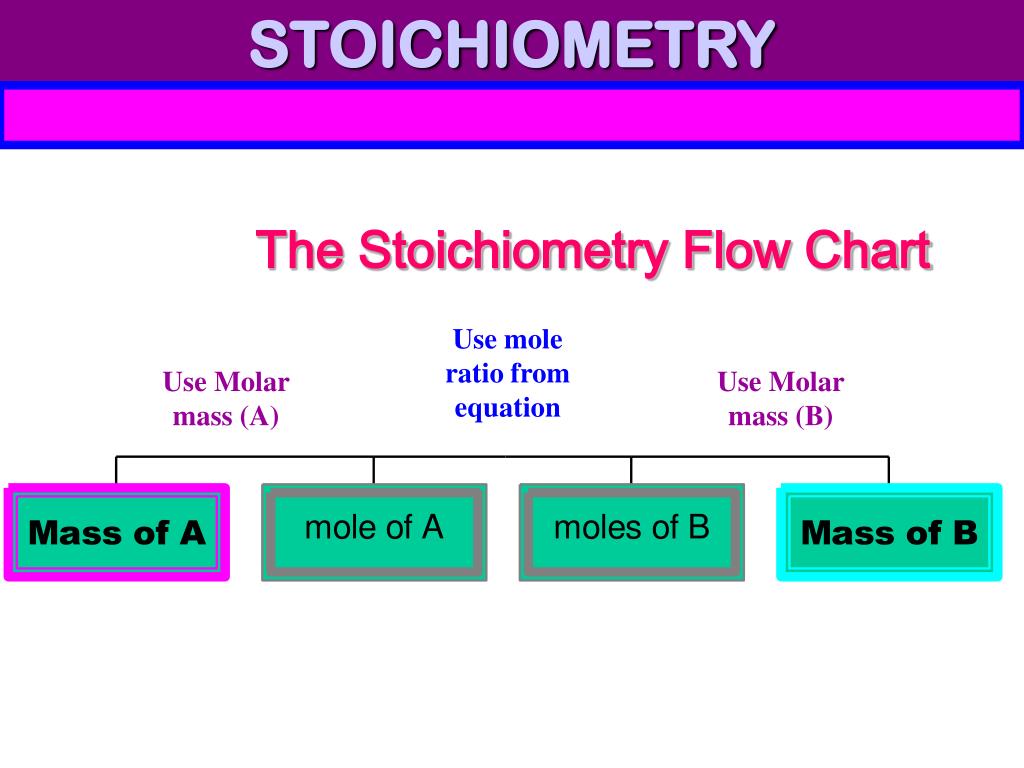
Stoichiometry Flow Chart
Stoichiometry Flow Chart - Perform stoichiometric calculations involving mass, moles, and solution molarity. Web derive chemical equations from narrative descriptions of chemical reactions. You must start with a balanced chemical equation. A stoichiometric quantity of a reactant is the amount necessary to react completely with the other reactant(s). Use balanced chemical equations to derive stoichiometric factors relating amounts of reactants and products. When chemists conduct experiments, they need to know how much of each reactant to use and what amount of product to expect. Web schematic flowchart showing how density is used for converting volume of pure substance to mass, molar mass for mass and moles, molarity for moles and volume of solution, avogadro's number for moles and number of particles, and stoichiometric factor for relating moles of one substance to another. Web explain the concept of stoichiometry as it pertains to chemical reactions. Flow chart showing three steps for using a balanced chemical equation to relate measured quantities of various reactants and products to each other. We will start with the simplest types of stoichiometric equations, those involving masses. Use balanced chemical equations to derive stoichiometric factors relating amounts of reactants and products; We will start with the simplest types of stoichiometric equations, those involving masses. Moles of b is converted to grams of b by the molar mass. Use creately’s easy online diagram editor to edit this diagram, collaborate with others and export results to multiple image formats. Web stoichiometry (/ ˌ s t ɔɪ k i ˈ ɒ m ɪ t r i /) is the relationship between the weights of reactants and products before, during, and following chemical reactions. Web at its core, stoichiometry is the study of the quantitative relationships between the reactants and products in chemical reactions. Web flowchart of steps in stoichiometric calculations. Perform stoichiometric calculations involving mass, moles, and solution molarity Web schematic flowchart showing how density is used for converting volume of pure substance to mass, molar mass for mass and moles, molarity for moles and volume of solution, avogadro's number for moles and number of particles, and stoichiometric factor for relating moles of one substance to another. Web stoichiometry is a general term for relationships between amounts of substances in chemical reactions. Web explain the concept of stoichiometry as it pertains to chemical reactions. Web outline of stoichiometry of chemical reactions. Writing and balancing chemical equations; Web covers the steps of basic conversions within stoichiometry without using dimensional analysis. Perform stoichiometric calculations involving mass, moles, and solution molarity Use balanced chemical equations to derive stoichiometric factors relating amounts of reactants and products; Use balanced chemical equations to derive stoichiometric factors relating amounts of reactants and products; Web explain the concept of stoichiometry as it pertains to chemical reactions. Web the stoichiometry of a reaction describes the relative amounts of reactants and products in a balanced chemical equation. Coefficients. We will start with the simplest types of stoichiometric equations, those involving masses. Perform stoichiometric calculations involving mass and moles. It also describes calculations done to determine how much of a substance will be used in a reaction, left over after a reaction, produced by a. You must start with a balanced chemical equation. Web the stoichiometry of a reaction. We can use these numerical relationships to write mole ratios, which allow us to convert between amounts of reactants and/or products (and thus solve stoichiometry problems!). Perform stoichiometric calculations involving mass, moles, and solution molarity. We will start with the simplest types of stoichiometric equations, those involving masses. You must start with a balanced chemical equation. Web stoichiometry (/ ˌ. Moles of a is converted to moles of b by multiplying by the molar ratio. Web explain the concept of stoichiometry as it pertains to chemical reactions. Determining how much of a specific substance can be created from a specific amount of another substance. Web explain the concept of stoichiometry as it pertains to chemical reactions; We can use these. Use balanced chemical equations to derive stoichiometric factors relating amounts of reactants and products; Perform stoichiometric calculations involving mass, moles, and solution molarity. Web explain the concept of stoichiometry as it pertains to chemical reactions; Web flow chart for solving stoichiometry problems: Perform stoichiometric calculations involving mass, moles, and solution molarity Use balanced chemical equations to derive stoichiometric factors relating amounts of reactants and products; Web stoichiometry molar mass the trick: Subscripts tell the number of atoms of each element in a molecule or compound. Web stoichiometry flow chart | creately. Web stoichiometry tutorial for converting mass and moles using stoichiometric conversions, balanced reactions, and molecular weights. A stoichiometric quantity of a reactant is the amount necessary to react completely with the other reactant(s). Determining how much of a specific substance can be created from a specific amount of another substance. Use balanced chemical equations to derive stoichiometric factors relating amounts of reactants and products; Coefficients tell the number of molecules or entities. Chose your starting point. Perform stoichiometric calculations involving mass, moles, and solution molarity Subscripts tell the number of atoms of each element in a molecule or compound. Web one can calculate the empirical formula from the percent composition. Web at its core, stoichiometry is the study of the quantitative relationships between the reactants and products in chemical reactions. Web stoichiometry tutorial for converting mass. We can use these numerical relationships to write mole ratios, which allow us to convert between amounts of reactants and/or products (and thus solve stoichiometry problems!). The flow chart depicts the various computational steps involved in most reaction stoichiometry calculations. Perform stoichiometric calculations involving mass and moles. Perform stoichiometric calculations involving mass, moles, and solution molarity. Write and balance chemical. Coefficients tell the number of molecules or entities. It also describes calculations done to determine how much of a substance will be used in a reaction, left over after a reaction, produced by a. You must start with a balanced chemical equation. Web at its core, stoichiometry is the study of the quantitative relationships between the reactants and products in chemical reactions. Grams of a is converted to moles by multiplying by the inverse of the molar mass. Web covers the steps of basic conversions within stoichiometry without using dimensional analysis. Use balanced chemical equations to derive stoichiometric factors relating amounts of reactants and products; Subscripts tell the number of atoms of each element in a molecule or compound. Web schematic flowchart showing how density is used for converting volume of pure substance to mass, molar mass for mass and moles, molarity for moles and volume of solution, avogadro's number for moles and number of particles, and stoichiometric factor for relating moles of one substance to another. You can easily edit this template using creately. Use balanced chemical equations to derive stoichiometric factors relating amounts of reactants and products; We will start with the simplest types of stoichiometric equations, those involving masses. Perform stoichiometric calculations involving mass and moles. Web flowchart of steps in stoichiometric calculations. Web one can calculate the empirical formula from the percent composition. Determining how much of a specific substance can be created from a specific amount of another substance.Stoichiometric Calculations CK12 Foundation
Extended Reaction Stoichiometry Road Map — Examples Expii
Stoichiometry CHEMISTRY HELP
Flow chart for Stoichiometry classroom Pinterest
stoichiometry flowchart introduction YouTube
PPT STOICHIOMETRY PowerPoint Presentation, free download ID4499255
PPT Chapter 12 Stoichiometry PowerPoint Presentation, free download
Stoichiometry Flow Chart
Stoichiometry Flow Chart YouTube
PPT STOICHIOMETRY PowerPoint Presentation, free download ID4499255
Web Subscripts And Coefficients Give Different Information.
Use Creately’s Easy Online Diagram Editor To Edit This Diagram, Collaborate With Others And Export Results To Multiple Image Formats.
Perform Stoichiometric Calculations Involving Mass, Moles, And Solution Molarity
A Stoichiometric Quantity Of A Reactant Is The Amount Necessary To React Completely With The Other Reactant(S).
Related Post: