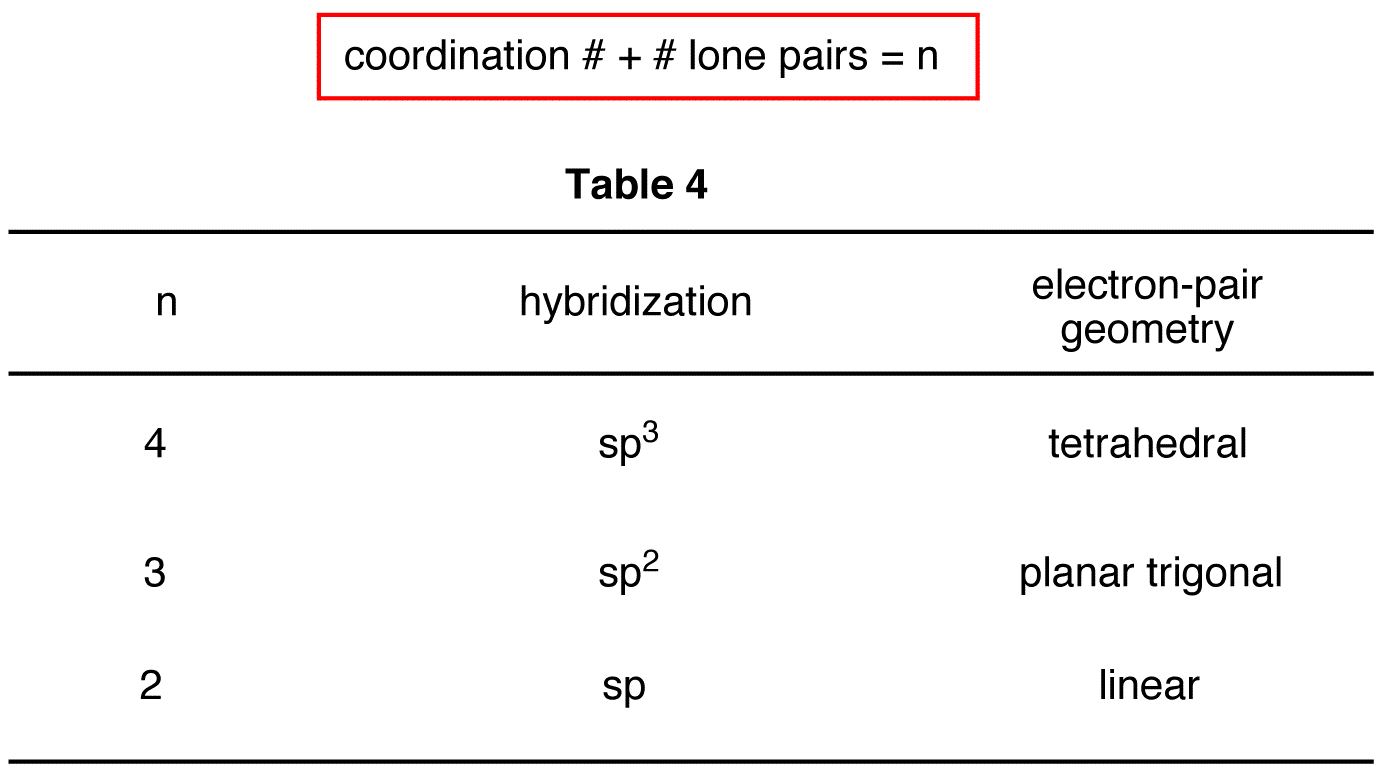
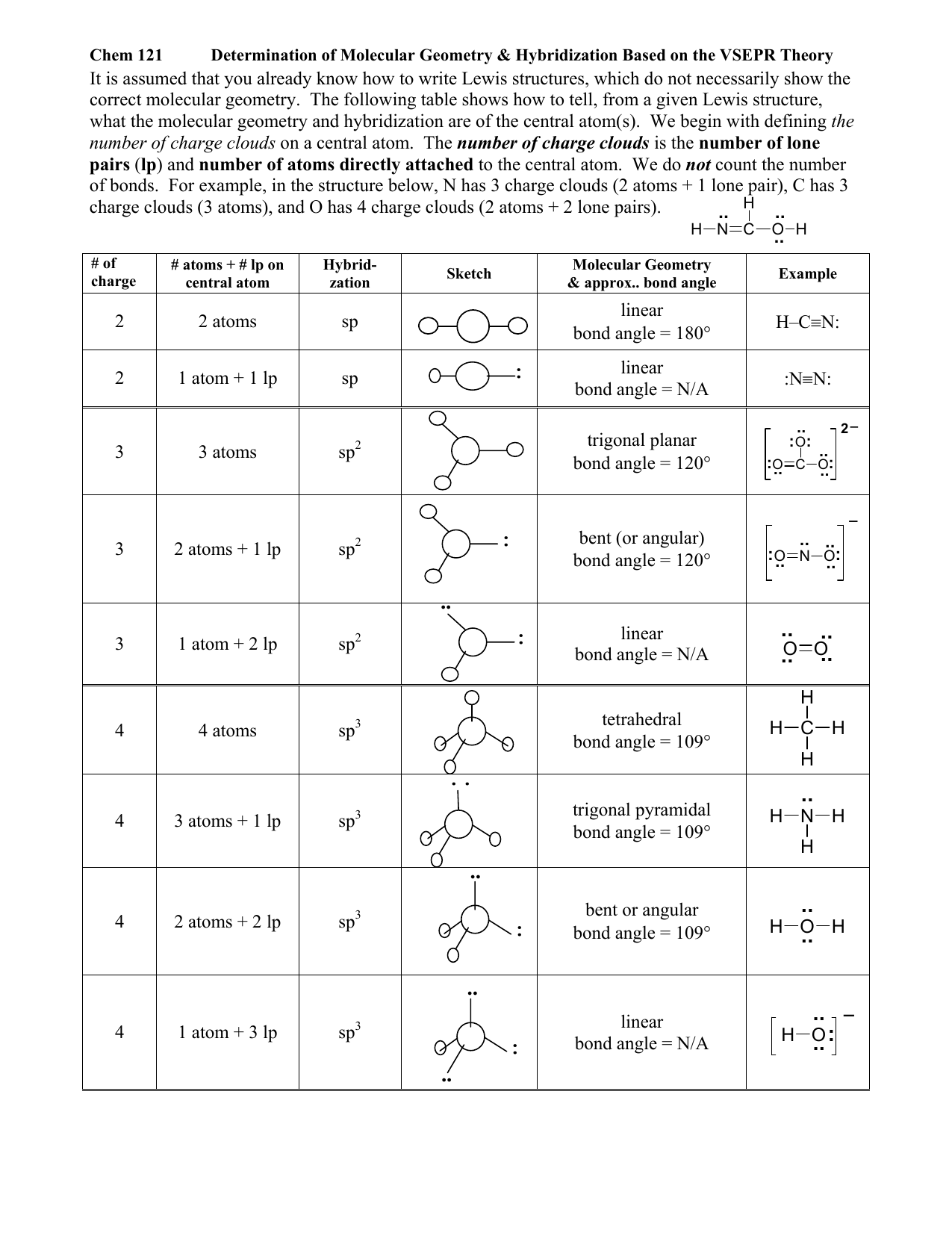
Molecular Geometry Chart With Hybridization
Molecular Geometry Chart With Hybridization - Do you know that several factors make atoms come together and combine to form several different chemical compounds? So, before we start with organic chemistry, let's revise a few things about bonding in organic molecules. Web in sp hybridization, one s orbital and one p orbital hybridize to form two sp orbitals, each consisting of 50% s character and 50% p character. Web h2s lewis structure. Web as we’ve seen, the ideal geometry for arranging four pairs of electrons is tetrahedral, which makes the hybridization of the central atom sp 3. The steric number is how many atoms are bonded to a central atom of a molecule plus the number of lone electron pairs attached to that atom. Sp 3 hybridization in methane. Covalent bonds are formed by: The valence shell electron pair repulsion (vsepr) theory is a simple and useful way to predict and rationalize the shapes of molecules. Where six are arranged, around each oxygen atom in a way that one side has four valence electrons. Sp 3 hybridization in methane. This 109.5 o arrangement gives tetrahedral geometry (figure 4). Web summary vsepr and hybridization table. Where six are arranged, around each oxygen atom in a way that one side has four valence electrons. Then, compare the model to real molecules! Lewis structures provide us with the number and types of bonds around a central atom, as well as any nb electron pairs. We will illustrate the use of this procedure with several examples, beginning with atoms with two electron groups. Determine the hybridization of the central atom ( 7 ). Usually, the electrons in the outermost shell of an atom, also known as valence electrons, take part in chemical. Geometric · blankets & throws · microwaves · pyrex · teenage engineering When atoms come together and create bonds, a new molecule is created. Find out by adding single, double or triple bonds and lone pairs to the central atom. Web this vsepr chart shows you all of the common vsepr geometries, organized by the steric number and how many lone electron pairs they have. Web board index chem 14a molecular shape. First and foremost it is important to determine how many valence electrons are present in the compound. Read ratings & reviewsshop best sellersshop our huge selectionfast shipping Usually, the electrons in the outermost shell of an atom, also known as valence electrons, take part in chemical. Web h2s lewis structure. Now to understand this we need to know the steps. These four valence electrons form two shared pairs of covalent bonds, providing a stable structure to the oxygen molecule. First and foremost it is important to determine how many valence electrons are present in the compound. Sp 3 hybridization in methane. The angle between adjacent bonds of an atom. Web this vsepr chart shows you all of the common vsepr. So, before we start with organic chemistry, let's revise a few things about bonding in organic molecules. Describes the arrangement of atoms around the central atom with acknowledgment to only bonding electrons. The following table shows the equivalence: Web as we’ve seen, the ideal geometry for arranging four pairs of electrons is tetrahedral, which makes the hybridization of the central. The steric number is how many atoms are bonded to a central atom of a molecule plus the number of lone electron pairs attached to that atom. Web explore molecule shapes by building molecules in 3d! Web board index chem 14a molecular shape and structure determining molecular shape (vsepr) Orbitals are combined in order to spread out electrons. The lewis. The following table shows the equivalence: Predict the actual geometry of the molecule or ion ( 6 ). Web we can find the hybridization of an atom in a molecule by either looking at the types of bonds surrounding the atom or by calculating its steric number. We will illustrate the use of this procedure with several examples, beginning with. The lewis diagram of o2 shows two oxygen atoms having twelve dots, of valence electrons. Where six are arranged, around each oxygen atom in a way that one side has four valence electrons. These four valence electrons form two shared pairs of covalent bonds, providing a stable structure to the oxygen molecule. In this video, we use both of these. Web board index chem 14a molecular shape and structure determining molecular shape (vsepr) Determine the hybridization of the central atom ( 7 ). The lewis diagram of o2 shows two oxygen atoms having twelve dots, of valence electrons. Web predict the electronic geometry using all areas of electron density (or, effective electron pairs) and the ideal bond angles associated with. In this compound valence electron will be as following: Predict the actual geometry of the molecule or ion ( 6 ). Web we can find the hybridization of an atom in a molecule by either looking at the types of bonds surrounding the atom or by calculating its steric number. The steric number is how many atoms are bonded to. Number of hybrid orbitals is equal to number of pure atomic orbitals used in the hybridization process. Determine the polarity of the molecule ( 8 ). Web we can find the hybridization of an atom in a molecule by either looking at the types of bonds surrounding the atom or by calculating its steric number. In this compound valence electron. Then, compare the model to real molecules! The angle between adjacent bonds of an atom. Where six are arranged, around each oxygen atom in a way that one side has four valence electrons. The lewis structure of h2s is as below. Web predict the electronic geometry using all areas of electron density (or, effective electron pairs) and the ideal bond angles associated with this geometry ( 5 ). Covalent bonds are formed by: The following table shows the equivalence: In this compound valence electron will be as following: How does molecule shape change with different numbers of bonds and electron pairs? We will illustrate the use of this procedure with several examples, beginning with atoms with two electron groups. Now to understand this we need to know the steps to draw a lewis structure at first. Web as we’ve seen, the ideal geometry for arranging four pairs of electrons is tetrahedral, which makes the hybridization of the central atom sp 3. First and foremost it is important to determine how many valence electrons are present in the compound. When atoms come together and create bonds, a new molecule is created. These four valence electrons form two shared pairs of covalent bonds, providing a stable structure to the oxygen molecule. Orbitals are combined in order to spread out electrons.MariePreAPChem Hybridization
C2H4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram
SO42 Lewis Structure, Molecular Geometry, Hybridization, and Polarity
Molecular Geometry And Hybridization Chart
VSEPR Chart With Hybridization
Molecular Geometry Chart With Hybridization
Xecl4 Lewis Structure Geometry Hybridization And Polarity guidetech
HYBRIDIZATION Teaching chemistry, Chemistry basics, Organic chemistry
Hybridization and Hybrid Orbitals ChemTalk
No3 Lewis Structure Molecular Geometry And Hybridization
Reactions Involve Making And Breaking Of Bonds!
Web Hybridization Of An S Orbital With All Three P Orbitals (P X , P Y, And P Z) Results In Four Sp 3 Hybrid Orbitals.
Web Lewis Structure Of O2.
Predicted Bond Angle(S) Hybridization Of Central Atom.
Related Post:



2]%2B%2B4.%2BTetrahedral.%2Bsp3.%2B[Cd(NH3)4]2%2B.jpg)


