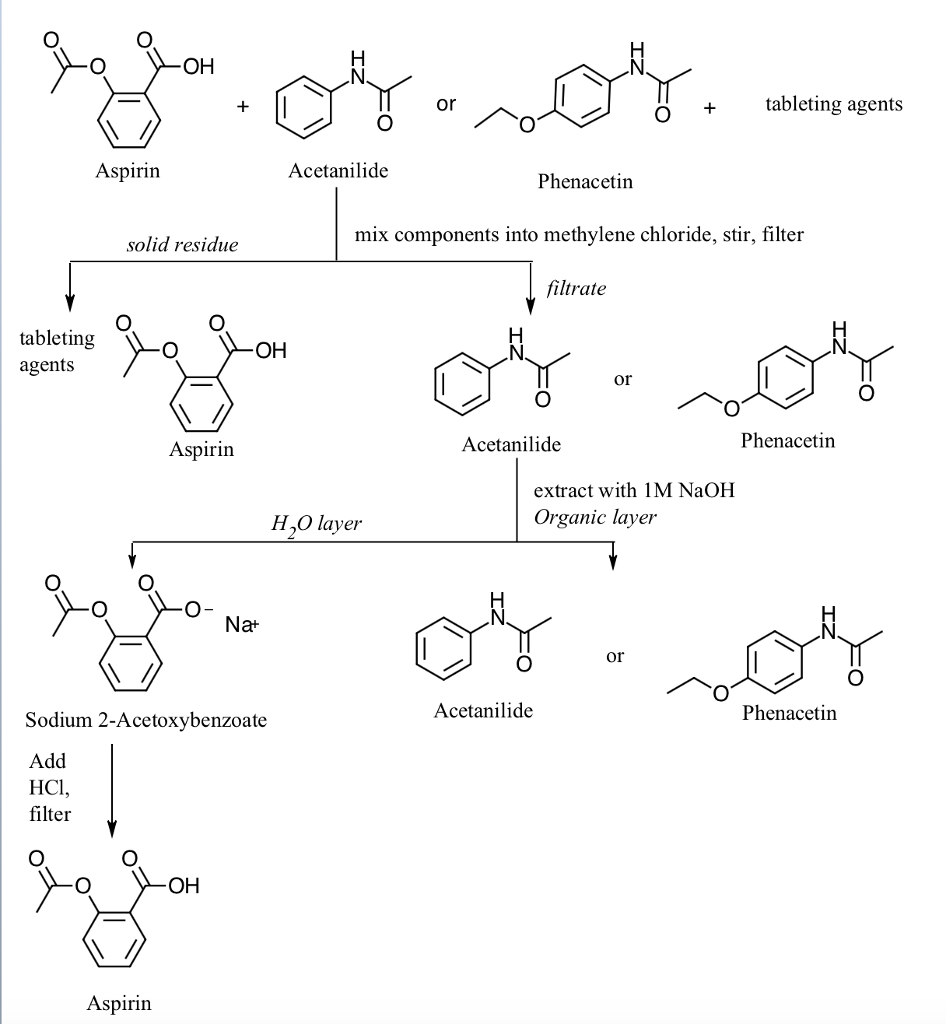
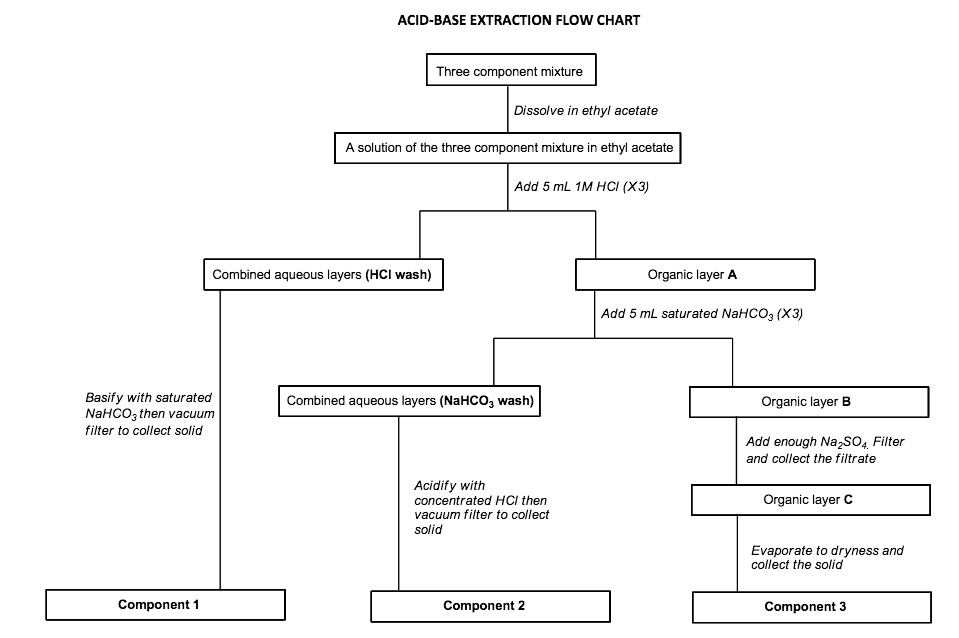
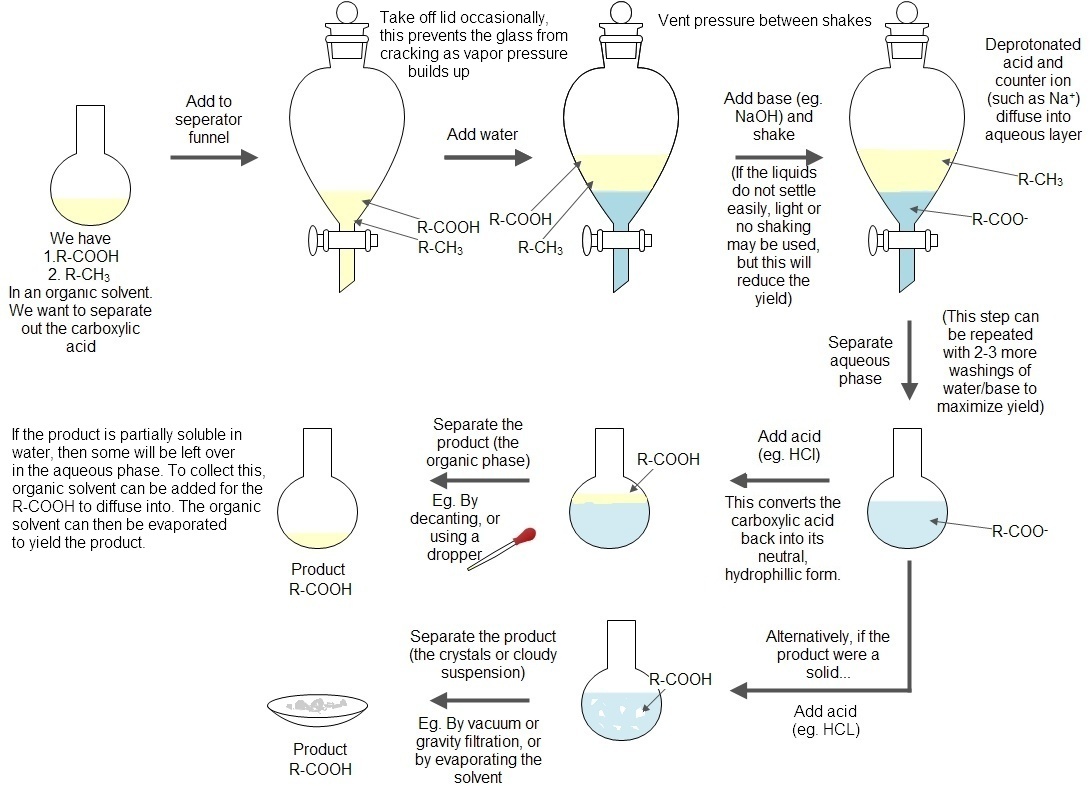
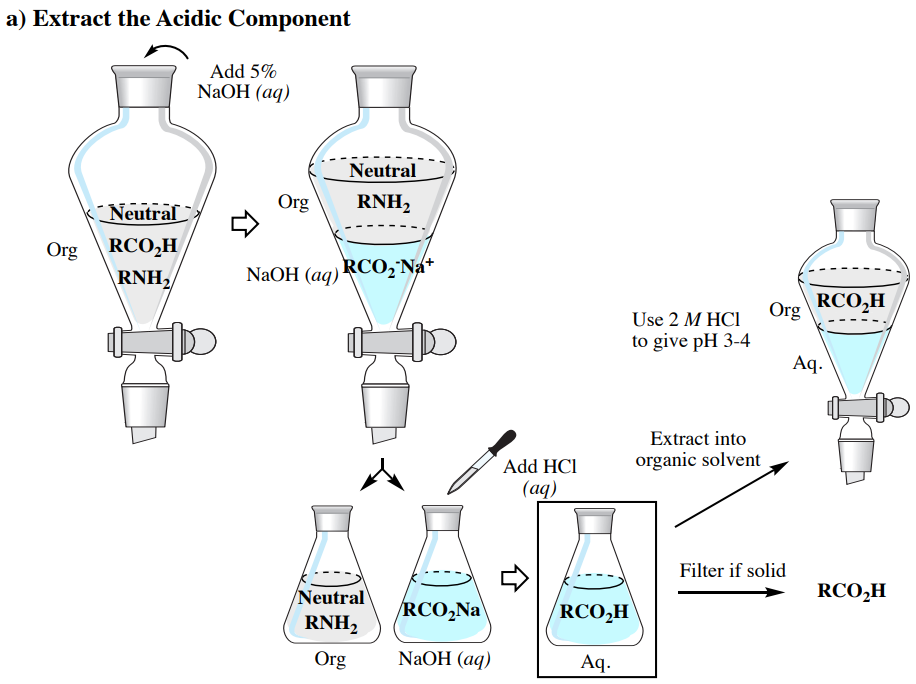
Acid Base Extraction Flow Chart
Acid Base Extraction Flow Chart - 20 ml of 10% bicarbonate is added to the solution in a separatory funnel where the aqueous There are three basic types of extraction: Web the flow chart on the next page outlines a general procedure for separating acidic, basic and neutral organic compounds using the principles of the solubility switch. Web this flow chart should provide a clear outline of all the steps in the process and should therefore enable you to follow the experiment more easily. Attach your small metal ring to one of the vertical rods on your rack 2. [study aids] combined acidic extract/combined basic extract [study aids] Web two base extraction introduction : It typically involves different solubility levels in water and an organic solvent. Common ones are ether, ethyl acetate, or dichloromethane. Web extraction and filtration can separate compounds based on their solubility properties. In this lab, you'll separate a mixture of cellulose, caffeine, and benzoic acid based on their solubilities in dichloromethane, or dcm, and water. Web extraction and filtration can separate compounds based on their solubility properties. 20 ml of 10% bicarbonate is added to the solution in a separatory funnel where the aqueous The general flowchart of the separation is shown below. There are three basic types of extraction: The acid and base chart is a reference table designed to make determining the strength of acids and bases simpler. Web via and acid/base reaction the changes in charge and polarity upon reaction for these species can be used to separate them due to their changes in solubility. This chart is ideal for use in the lab or in the classroom. Web however, by taking advantage of the presence of acidic and basic groups, it is sometimes possible to achieve clean separations of mixtures using a separating funnel, an organic solvent such as ether, and a sequence of extractions with strong acids and bases. Attach your small metal ring to one of the vertical rods on your rack 2. Attach your small metal ring to one of the vertical rods on your rack 2. Attach your small metal ring to one of the vertical rods on your rack 2. Separation of a neutral from a carboxylic acid. Web acid/base extraction flow chart. This chart is ideal for use in the lab or in the classroom. The acid and base chart is a reference table designed to make determining the strength of acids and bases simpler. There are three basic types of extraction: Web however, by taking advantage of the presence of acidic and basic groups, it is sometimes possible to achieve clean separations of mixtures using a separating funnel, an organic solvent such as ether,. This chart is ideal for use in the lab or in the classroom. Web via and acid/base reaction the changes in charge and polarity upon reaction for these species can be used to separate them due to their changes in solubility. Of acid rco 2 h rco 2 h acid rnh 2 base n neutral extract with 5% naoh organic. The general flowchart of the separation is shown below. Common ones are ether, ethyl acetate, or dichloromethane. Web extraction and filtration can separate compounds based on their solubility properties. The mixture is dissolved in 30 ml of diethyl ether. Web we wash the organic reaction mixture with water (acidic, neutral and/or basic) to remove any byproducts or inorganic material. Web two base extraction introduction : We will be performing the first two types of extraction in this experiment. The acid and base chart is a reference table designed to make determining the strength of acids and bases simpler. Web the flow chart on the next page outlines a general procedure for separating acidic, basic and neutral organic compounds using. Web extraction and filtration can separate compounds based on their solubility properties. We will be performing the first two types of extraction in this experiment. This chart is ideal for use in the lab or in the classroom. Web via and acid/base reaction the changes in charge and polarity upon reaction for these species can be used to separate them. Attach your small metal ring to one of the vertical rods on your rack 2. It typically involves different solubility levels in water and an organic solvent. Web this flow chart should provide a clear outline of all the steps in the process and should therefore enable you to follow the experiment more easily. Separation of a neutral from a. Web chem 344 extraction flowchart. Web this flow chart should provide a clear outline of all the steps in the process and should therefore enable you to follow the experiment more easily. Web flow chart of acid base extraction. Attach your small metal ring to one of the vertical rods on your rack 2. Web extraction and filtration can separate. Web this flow chart should provide a clear outline of all the steps in the process and should therefore enable you to follow the experiment more easily. Web acid/base extraction flow chart. Web figure 6 details a full acid/base extraction flow chart that describes the separation of a mixture of an organic acid (ha), organic base (b:) and a neutral. Web figure 6 details a full acid/base extraction flow chart that describes the separation of a mixture of an organic acid (ha), organic base (b:) and a neutral organic molecule (n). Web flow chart of acid base extraction. In this lab, you'll separate a mixture of cellulose, caffeine, and benzoic acid based on their solubilities in dichloromethane, or dcm, and. It typically involves different solubility levels in water and an organic solvent. 20 ml of 10% bicarbonate is added to the solution in a separatory funnel where the aqueous Web acid/base extraction flow chart. Separation of a neutral from a carboxylic acid. Web this flow chart should provide a clear outline of all the steps in the process and should therefore enable you to follow the experiment more easily. Do not ignore this requirement. [study aids] combined acidic extract/combined basic extract [study aids] There are three basic types of extraction: The acid and base chart is a reference table designed to make determining the strength of acids and bases simpler. In this lab, you'll separate a mixture of cellulose, caffeine, and benzoic acid based on their solubilities in dichloromethane, or dcm, and water. Web two base extraction introduction : Web we wash the organic reaction mixture with water (acidic, neutral and/or basic) to remove any byproducts or inorganic material. We will be performing the first two types of extraction in this experiment. Web the flow chart on the next page outlines a general procedure for separating acidic, basic and neutral organic compounds using the principles of the solubility switch. Web however, by taking advantage of the presence of acidic and basic groups, it is sometimes possible to achieve clean separations of mixtures using a separating funnel, an organic solvent such as ether, and a sequence of extractions with strong acids and bases. Aspirin (acetlysalicylic acid) dissolve mixture in about 30ml dichloromethane in erlenmeyer flask and transfer dissolved solution into a separatory funnel.Acid Base Extraction Flow Chart
Acid Base Extraction Flow Chart
ACIDBASE EXTRACTION FLOW CHARTThree component mixtur… SolvedLib
Acid Base Extraction Flow Chart
Acidbase Extraction Flow Chart
Extraction Flow Chart Organic Chemistry
Visually Tracking AcidBase Extractions Using Colorful Compounds
Flow Chart Of Acid Base Extraction flow chart
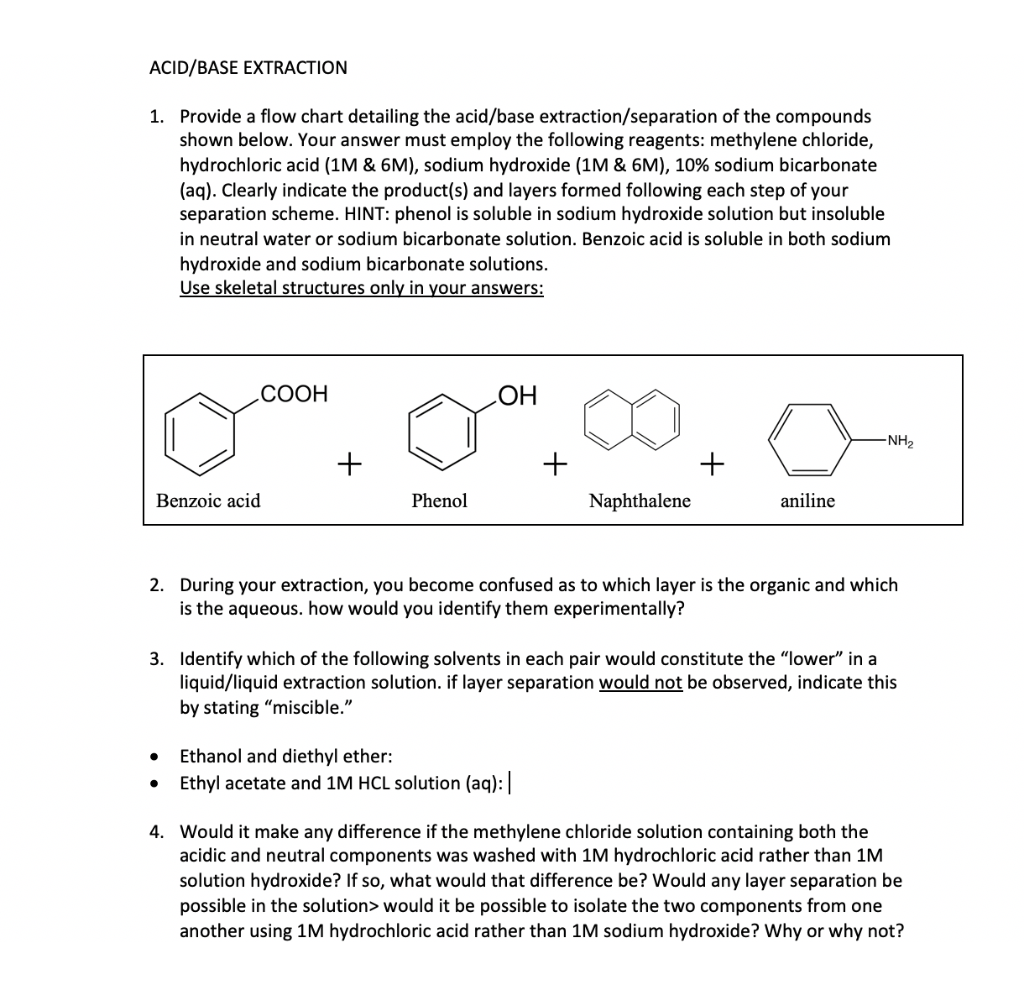
Solved ACID/BASE EXTRACTION Provide a flow chart detailing
Acid Base Flowchart
Web Figure 6 Details A Full Acid/Base Extraction Flow Chart That Describes The Separation Of A Mixture Of An Organic Acid (Ha), Organic Base (B:) And A Neutral Organic Molecule (N).
This Chart Is Ideal For Use In The Lab Or In The Classroom.
Web Extraction And Filtration Can Separate Compounds Based On Their Solubility Properties.
Separation Of A Neutral From A Carboxylic Acid.
Related Post: